Pipeline
You can scroll left and right to view the full content.
| Pipeline | Development Projects | Target diseases | Development of drug candidate | Safety test | Preclinical trials | Clinical trial - phase 1 | Clinical trial - phase 2 |
|---|---|---|---|---|---|---|---|
| FT005 | FT005-002 (Hulk) | Chronic kidney disease |
|
||||
| Sarcopenia (muscle atrophy with aging) |
|
||||||
| Muscular dystrophy |
|
||||||
| FT006 | FT006-001 (Batman) | COVID prevention |
|
||||
| FT007 | FT005-002 (Hulk) | Cancer cachexia |
|
||||
| FT005-007 (Steve) |
|
||||||
| FT009 | FT005-002 (Hulk) | Type 2 diabetes |
|
||||
| FT011 | FT005-005 (Paul) | Melanoma (skin cancer) |
|
||||
| FT012 | FT005-006 (Kyte) | Melanoma (skin cancer) |
|
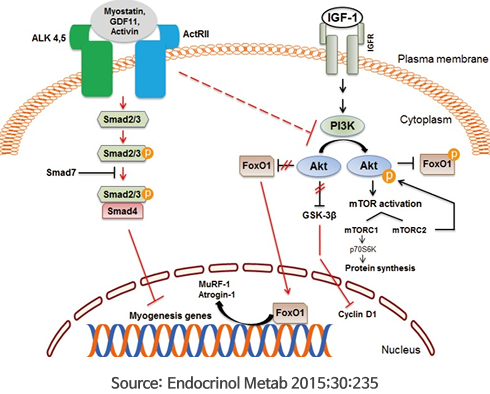
FT005-002 (Hulk) Project (Target antigen: GDF-8, myostain)
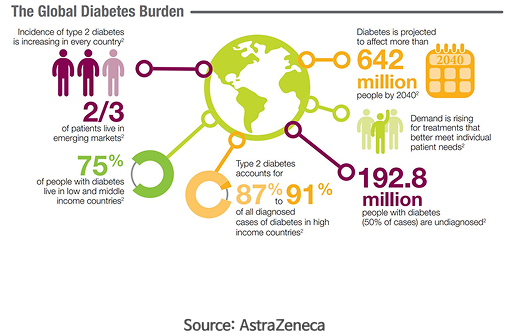
Type 2 diabetes immunotherapy
| Mechanism of action |
|
|---|---|
| Development progress |
|
| Market size |
|
| Competitors and their products |
|
Type 2 diabetes immunotherapy commercialization plan
| Prospective technology recipient | Target milestone | Total technology transfer target fee | Remarks (including terms of agreement) |
|---|---|---|---|
| Big Pharma | Phase 1 IND approval | KRW 9 billion (1% of the market) | Royalty: 5% of revenue |
| Big Pharma | Phase 2 approval | KRW 1.8 trillion (2% of the market) | Royalty: 10% of revenue |
Cookie notice
By clicking 'Accept all cookies', you agree to the storing of cookies on your device and to the associated processing of data to enhance site navigation, analyse site usage, and assist in our marketing and performance efforts.
Cookie Consent Settings
- Strictly Necessary Cookies
- Functional Cookies
- Marketing/Advertising Cookies
- Statistics Cookies