Pipeline
You can scroll left and right to view the full content.
| Pipeline | Development Projects | Target diseases | Development of drug candidate | Safety test | Preclinical trials | Clinical trial - phase 1 | Clinical trial - phase 2 |
|---|---|---|---|---|---|---|---|
| FT005 | FT005-002 (Hulk) | Chronic kidney disease |
|
||||
| Sarcopenia (muscle atrophy with aging) |
|
||||||
| Muscular dystrophy |
|
||||||
| FT006 | FT006-001 (Batman) | COVID prevention |
|
||||
| FT007 | FT005-002 (Hulk) | Cancer cachexia |
|
||||
| FT005-007 (Steve) |
|
||||||
| FT009 | FT005-002 (Hulk) | Type 2 diabetes |
|
||||
| FT011 | FT005-005 (Paul) | Melanoma (skin cancer) |
|
||||
| FT012 | FT005-004 (Kyte) | Melanoma (skin cancer) |
|
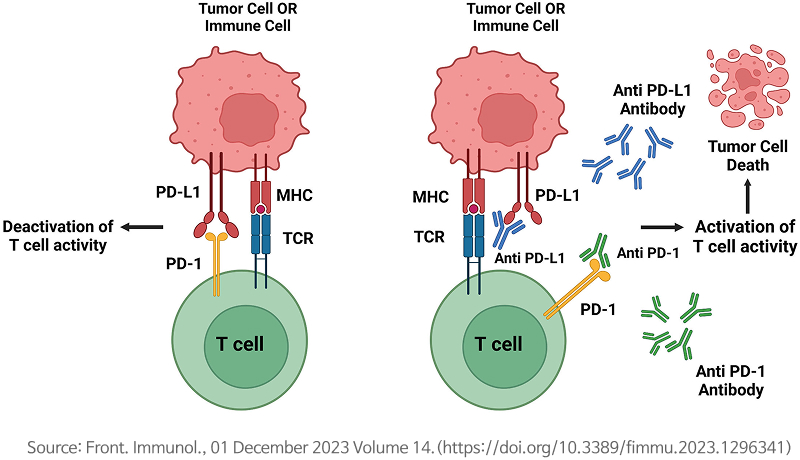
FT005-004 (Kyte) project (target antigen: B7-H1 (PD-L1))
Mechanism of B7-H1 (PD-L1) antibody therapy
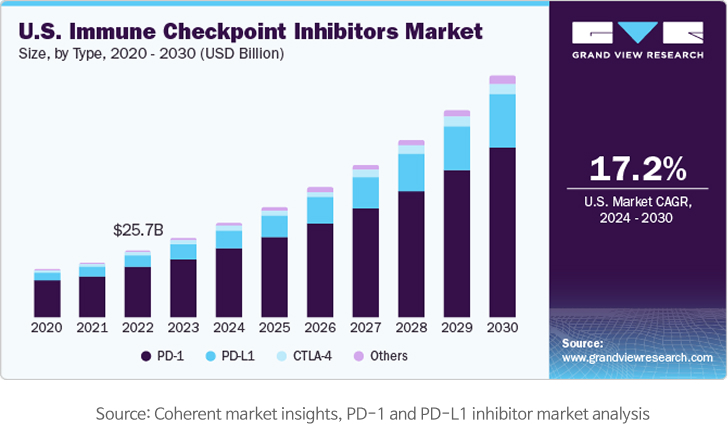
Market size
The overall immune checkpoint inhibitor market, including PD-1/PD-L1 inhibitors, was estimated at USD 48.42 billion as of 2023. With a CAGR of 17.9% from 2024 to 2030, the market is expected to reach USD 154.25 billion by 2030. In addition, the PD-L1 inhibitor market alone is expected to take up roughly 22% of the total immune checkpoint inhibitor market, reaching around USD 35 billion by 2030.
Cookie notice
By clicking 'Accept all cookies', you agree to the storing of cookies on your device and to the associated processing of data to enhance site navigation, analyse site usage, and assist in our marketing and performance efforts.
Cookie Consent Settings
- Strictly Necessary Cookies
- Functional Cookies
- Marketing/Advertising Cookies
- Statistics Cookies